Glaukos iStent® for those of you who have glaucoma and cataract
If you have co-existent cataract and glaucoma then this could be the ideal option for you. Glaucoma is caused by obstruction/resistance to the drainage of the
clear fluid (aqueous) from inside the eye. As the eye in non-distensible (cannot stretch) any increase in the amount of fluid in the eye can send the pressure high and damage the optic nerve. The iStent is inserted at the time of cataract surgery and bypasses the area of resistance to fluid outflow. It aims to get the pressure down in the eye and hopefully allow us to reduce the number of (or even stop) your glaucoma eye drops.
This additional procedure extends the operation by only a few minutes and the benefits can be long lasting.
The iStent® is the smallest medical device that has ever been implanted in the human body
- Approximately 1 mm in length
- Size: 0.5 mm x 0.25 mm x 1.0 mm
- Weight: approximately 60 µg
- Snorkel bore diameter: 120 µm
- Made of surgical grade nonferromagnetic titanium


iStent® Surgical Procedure
The iStent® is inserted through the clear, cornea cataract incision and can be performed under local or topical anesthesia.

The more technical stuff:
iStent® bypasses the trabecular meshwork in the infranasal locations
- Maximizing outflow through Schlemm's canal
- Reestablishing continuous, physiological outflow in the lower nasal quadrants
- Increasing outflow due to the high presence of collector channel congregations[3, 5]
iStent® Maximizes Outflow Through One Opening

Reduce or Eliminate Medication with iStent®
Improve IOP control in more patients with iStent®
Percentage of Patients Who Sustained IOP ≤21 mm Hg without Medication Use
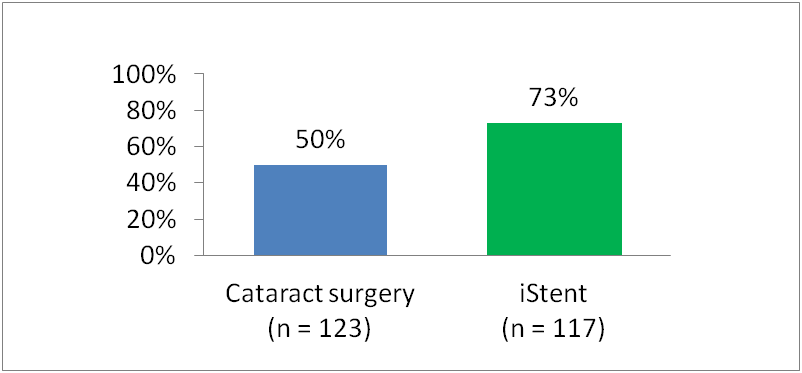
Prospective, randomized, multicenter study across 27 US sites, of 240 patients with cataract and mild to moderate glaucoma were treated with ocular antihypertensive medications at baseline. All patients were assigned to a postmedication washout period before undergoing cataract surgery or iStent® implantation plus cataract surgery.
At 12 months
- 73% of patients who received iStent® remained medication free while sustaining target IOPs of ≤21 mm Hg versus only 50% of those who underwent cataract surgery alone (P<0.001)
Percentage of Patients who Sustained a ≥20% Reduction in IOP Without Medication Use
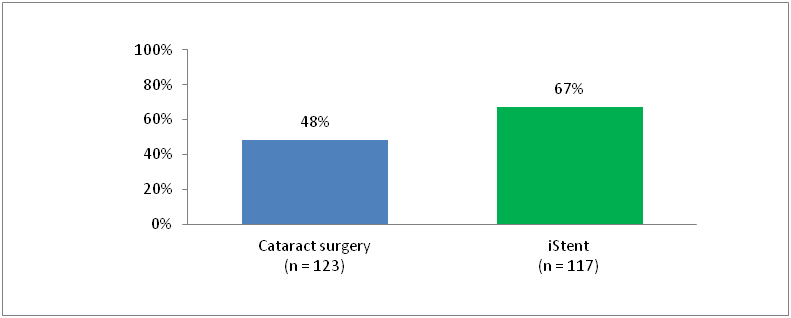
At 12 months
- 67% of patients who received iStent® remained medication free while sustaining a mean IOP reduction of ≥20% versus only 48% who underwent cataract surgery alone (P<0.002)
- 19% more patients who received iStent® sustained a ≥20% reduction in IOP without medication use versus cataract surgery alone
References:
1. Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM: Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res 1989, 8:1233-1240.
2. Spiegel D, Garcia-Feijoo J, Garcia-Sanchez J, Lamielle H: Coexistent primary open-angle glaucoma and cataract: preliminary analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent. Adv Ther 2008, 25:453-464.
3. Dvorak-Theobald G: Schlemm's Canal: Its Anastomoses and Anatomic Relations. Trans Am Ophthalmol Soc 1934, 32:574-595.
4. Bahler CK, Smedley GT, Zhou J, Johnson DH: Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am J Ophthalmol 2004, 138:988-994.
5. Zhou J, Smedley GT: Trabecular bypass: effect of schlemm canal and collector channel dilation. J Glaucoma 2006, 15:446-455.
6. Samuelson TW. Prospective Randomized Trial of Cataract Surgery with iStent Implantation and Cataract Surgery Alone in Mild-Moderate Open-Angle Glaucoma. Paper presented at: AAO Annual Meeting, October 27, 200; San Francisco.
7. Quigley HA, Broman AT: The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006, 90:262-267.
8. Lum F, Schein O, Schachat AP, Abbott RL, Hoskins HD, Jr., Steinberg EP: Initial two years of experience with the AAO National Eyecare Outcomes Network (NEON) cataract surgery database. Ophthalmology 2000, 107:691-697.
9. Konstas AG, Mikropoulos D, Dimopoulos AT, Moumtzis G, Nelson LA, Stewart WC: Second-line therapy with dorzolamide/timolol or latanoprost/timolol fixed combination versus adding dorzolamide/timolol fixed combination to latanoprost monotherapy. Br J Ophthalmol 2008, 92:1498-1502.
10. Fea AM: Results of phacoemulsification compared with phacoemulsification and micro-bypass stent implantation in patients with POAG: A Randomized, Double-Masked Clinical Trial. In the Annual Meeting of the American Academy of Ophthalmology; Atlanta, GA 2008
11. Cantor LB, Katz LJ, Cheng JW, Chen E, Tong KB, Peabody JW: Economic evaluation of medication, laser trabeculoplasty and filtering surgeries in treating patients with glaucoma in the US. Curr Med Res Opin 2008, 24:2905-2918.
12. McKinnon SJ, Goldberg LD, Peeples P, Walt JG, Bramley TJ: Current management of glaucoma and the need for complete therapy. Am J Manag Care 2008, 14:S20-27.
13. Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM: Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol 2005, 140:598-606.
14. Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B: Patient-reported behavior and problems in using glaucoma medications. Ophthalmology 2006, 113:431-436.
15. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol 2000, 130:429-440.
16. Noecker RJ, Herrygers LA, Anwaruddin R: Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea 2004, 23:490-496.
